Modern PRP: “Clinical PRP”
In the past 10 years, the treatment scheme of PRP has undergone great changes. Through experimental and clinical research, we now have a better understanding of platelet and other cell physiology. In addition, several high-quality systematic evaluations, meta-analyses and randomized controlled trials have shown the effectiveness of PRP biotechnology in many medical fields, including dermatology, cardiac surgery, plastic surgery, orthopedic surgery, pain management, spinal diseases, and sports medicine.
The current characteristic of PRP is its absolute platelet concentration, which changes from the initial definition of PRP (including platelet concentration higher than the baseline value) to more than 1 × 10 6/µ L or about 5 times the minimum platelet concentration in platelets from baseline. In the extensive review by Fadadu et al. 33 PRP systems and protocols were evaluated. The platelet count of the final PRP preparation produced by some of these systems is lower than that of the whole blood. They reported that the platelet factor of PRP increased as low as 0.52 with the single spin kit (Selphyl ®). In contrast, double-rotation EmCyte Genesis PurePRPII ® The platelet concentration produced by the device is the highest (1.6 × 10 6 /µL) .
Obviously, in vitro and animal methods are not the ideal research environment for successful transformation into clinical practice. Similarly, the device comparison study does not support the decision, because they show that the platelet concentration between PRP devices is very different. Fortunately, through proteomics based technology and analysis, we can increase our understanding of the cell functions in PRP that affect the treatment results. Before reaching consensus on standardized PRP preparations and formulations, PRP should follow clinical PRP formulations to promote substantial tissue repair mechanisms and progressive clinical results.
Clinical PRP formula
At present, effective clinical PRP (C-PRP) has been characterized as a complex composition of autologous multicellular components in small volume plasma obtained from a part of peripheral blood after centrifugation. After centrifugation, PRP and its non-platelet cell components can be recovered from the concentration device according to different cell densities (of which the platelet density is the lowest).
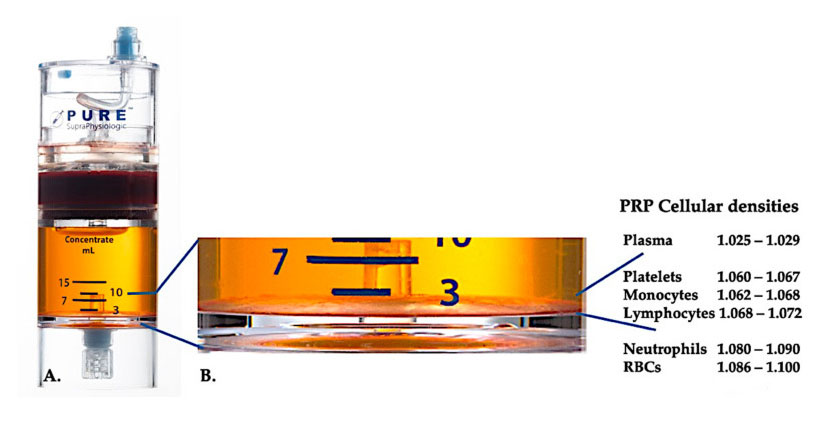
Use PurePRP-SP ® Cell density separation equipment (EmCyte Corporation, Fort Myers, FL, USA) was used for whole blood after two centrifugation procedures. After the first centrifugation process, the whole blood component was separated into two basic layers, platelet (lean) plasma suspension and red blood cell layer. In A, the second centrifugation step has been completed. The actual PRP volume can be extracted for patient application. The magnification in B shows that there is organized multi-component erythrocyte sedimentation brown layer (represented by blue line) at the bottom of the equipment, which contains high concentrations of platelets, monocytes and lymphocytes, based on the density gradient. In this example, according to the C-PRP preparation protocol with poor neutrophils, the minimum percentage of neutrophils (<0.3%) and erythrocytes (<0.1%) will be extracted.
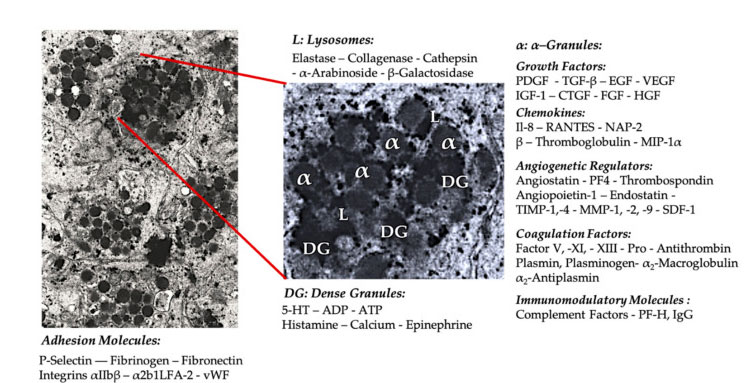
Platelet granule
In the early clinical PRP application, α- Granules are the most commonly cited platelet internal structure, because they contain coagulation factors, a large number of PDGF and angiogenic regulators, but have little thrombogenic function. Other factors include less well-known chemokine and cytokine components, such as platelet factor 4 (PF4), pre-platelet basic protein, P-selectin (an activator of integrin) and chemokine RANTES (regulated by activation, expressing normal T cells and presumably secreting). The overall function of these specific platelet granule components is to recruit and activate other immune cells or induce endothelial cell inflammation.
Dense granular components such as ADP, serotonin, polyphosphate, histamine and adrenaline are more implicitly used as regulators of platelet activation and thrombosis. Most importantly, many of these elements have the function of modifying immune cells. Platelet ADP is recognized by P2Y12ADP receptor on dendritic cells (DC), thus increasing antigen endocytosis. DC (antigen presenting cell) is very important for initiating T cell immune response and controlling protective immune response, which links the innate immune system and adaptive immune system. In addition, platelet adenosine triphosphate (ATP) sends signals through T cell receptor P2X7, leading to increased differentiation of CD4 T helper cells into proinflammatory T helper 17 (Th17) cells. Other platelet dense granule components (such as glutamate and serotonin) induce T cell migration and increase monocyte differentiation to DC, respectively. In PRP, these immunomodulators derived from dense particles are highly enriched and have substantial immune functions.
The number of direct and indirect potential interactions between platelets and other (receptor) cells is extensive. Therefore, the application of PRP in the local pathological tissue environment can induce a variety of inflammatory effects.
Platelet concentration
C-PRP should contain clinical doses of concentrated platelets to produce beneficial therapeutic effects. Platelets in C-PRP should stimulate cell proliferation, the synthesis of mesenchymal and neurotrophic factors, promote the migration of chemotactic cells and stimulate immunoregulatory activity, as shown in the figure.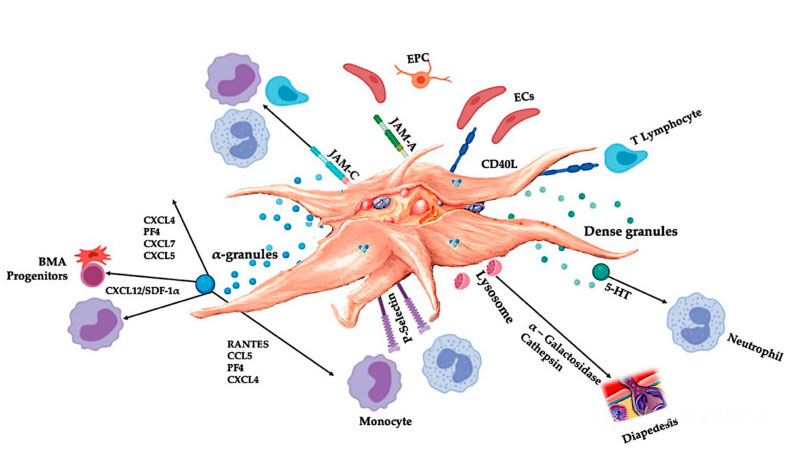
Activated platelets, release of PGF and adhesion molecules mediate a variety of cell interactions: chemotaxis, cell adhesion, migration, and cell differentiation, and regulate immune regulatory activities. These platelet cell-cell interactions contribute to angiogenesis and inflammatory activity, and ultimately stimulate the tissue repair process. Abbreviations: BMA: bone marrow aspirate, EPC: endothelial progenitor cells, EC: endothelial cells, 5-HT: 5-hydroxytryptamine, RANTES: activated regulation of normal T cell expression and putative secretion, JAM: junction adhesion molecule type, CD40L: cluster 40 ligand, SDF-1 α: Stromal cell-derived factor-1 α, CXCL: chemokine (CXC motif) ligand, PF4: platelet factor 4. Adapted from Everts et al.
Marx was the first person to prove that bone and soft tissue healing was enhanced, and the minimum platelet count was 1 × 10 6 /µL。 These results were confirmed in a study of lumbar fusion through intervertebral foramen, when the platelet dose was greater than 1.3 × At 106 platelets/µ L, this study demonstrated more fusion. In addition, Giusti et al. Revealed 1.5 × The tissue repair mechanism at a dose of 109 requires platelets/mL to induce functional angiogenesis through endothelial cell activity. In the latter study, higher concentrations reduced the angiogenesis potential of platelets in and around follicles. In addition, earlier data showed that the dose of PRP would also affect the treatment results. Therefore, in order to significantly induce angiogenesis reaction and stimulate cell proliferation and cell migration, C-PRP should contain at least 7.5 in a 5-mL PRP treatment bottle × 10 9 can deliver platelets.
In addition to dose dependence, the effect of PRP on cell activity seems to be highly time-dependent. Sophie et al. These results suggest that short-term exposure to human platelet lysates can stimulate bone cell proliferation and chemotaxis. On the contrary, long-term exposure to PRP will lead to lower levels of alkaline phosphatase and mineral formation.
Red blood cell
Red blood cells are responsible for transporting oxygen to the tissues and transferring carbon dioxide from the tissues to the lungs. They have no nucleus and are composed of heme molecules that bind to proteins. The iron and heme components in red blood cells promote the combination of oxygen and carbon dioxide. Generally, the life cycle of red blood cells is about 120 days. They are removed from the circulation by macrophages through a process called RBC aging. Red blood cells in PRP samples may be damaged under shear conditions (for example, whole-blood bleeding surgery, immune-mediated process, oxidative stress or inadequate PRP concentration scheme). Therefore, RBC cell membrane decomposes and releases toxic hemoglobin (Hb), measured by plasma free hemoglobin (PFH), heme and iron.]. PFH and its degradation products (heme and iron) jointly lead to harmful and cytotoxic effects on tissues, leading to oxidative stress, loss of nitric oxide, activation of inflammatory pathways and immunosuppression. These effects will eventually lead to microcirculation dysfunction, local vasoconstriction and vascular injury, as well as serious tissue damage.
The most important thing is that when RBC containing C-PRP is delivered to the tissue, it will cause a local reaction called eryptosis, which will trigger the release of an effective cytokine and macrophage migration inhibitor. This cytokine inhibits the migration of monocytes and macrophages. It exerts strong pro-inflammatory signals to surrounding tissues, inhibits stem cell migration and fibroblast proliferation, and leads to significant local cell dysfunction. Therefore, it is important to limit RBC contamination in PRP preparations. In addition, the role of red blood cells in tissue regeneration has never been determined. Adequate C-PRP centrifugation and preparation process will usually reduce or even eliminate the presence of red blood cells, thus avoiding the adverse consequences of hemolysis and polycythemia.
Leukocytes in C-PRP
The presence of white blood cells in PRP preparations depends on the treatment equipment and preparation scheme. In plasma-based PRP equipment, white blood cells are completely eliminated; However, white blood cells were significantly concentrated in the PRP preparation of erythrocyte sedimentation brown layer. Due to its immune and host defense mechanisms, white blood cells greatly affect the internal biology of acute and chronic tissue conditions. These features will be discussed further below. Therefore, the presence of specific leukocytes in C-PRP can cause significant cellular and tissue effects. More specifically, different PRP erythrocyte sedimentation brown-yellow layer systems use different preparation schemes, thus producing different proportion of neutrophils, lymphocytes and monocytes in PRP. Eosinophils and basophils cannot be measured in PRP preparations because their cell membranes are too fragile to withstand centrifugal processing forces.
Neutrophils
Neutrophils are essential leukocytes in many healing pathways. These pathways combine with antimicrobial proteins present in platelets to form a dense barrier against invasive pathogens. The existence of neutrophils is determined according to the treatment target of C-PRP. Increased levels of tissue inflammation may be required in chronic wound care PRP biotherapy or in applications aimed at bone growth or healing. Importantly, additional neutrophil functions have been found in several models, emphasizing their role in angiogenesis and tissue repair. However, neutrophils can also cause harmful effects, so they are not suitable for some applications. Zhou and Wang proved that the use of PRP rich in neutrophils can lead to an increase in the ratio of type III collagen to type I collagen, thus exacerbating fibrosis and reducing tendon strength. Other harmful characteristics mediated by neutrophils are the release of inflammatory cytokines and matrix metalloproteinases (MMPs), which can promote inflammation and catabolism when applied to tissues.
Leukomonocyte
In C-PRP, mononuclear T and B lymphocytes are more concentrated than any other white blood cells. They are closely related to cell-mediated cytotoxic adaptive immunity. Lymphocytes can trigger cell reactions to fight infection and adapt to invaders. In addition, T-lymphocyte derived cytokines (interferon- γ [IFN- γ] And interleukin-4 (IL-4) enhance the polarization of macrophages. Verassar et al. It is proved that conventional T lymphocytes can indirectly promote the tissue healing in the mouse model by regulating the differentiation of monocytes and macrophages.
Monocyte – multipotent repair cell
According to the PRP preparation device used, monocytes may protrude or not exist in the PRP treatment bottle. Unfortunately, their performance and regeneration ability are rarely discussed in the literature. Therefore, little attention is paid to monocytes in the preparation method or final formula. Monocyte group is heterogeneous, originating from progenitor cells in bone marrow, and transported to peripheral tissues through hematopoietic stem cell pathway according to microenvironment stimulation. During homeostasis and inflammation, circulating monocytes leave the blood stream and are recruited to injured or degraded tissues. They can act as macrophages (M Φ) Effector cells or progenitor cells. Monocytes, macrophages and dendritic cells represent the mononuclear phagocytic system (MPS).. A typical feature of MPS is the plasticity of its gene expression pattern and the functional overlap between these cell types. In degenerated tissues, resident macrophages, locally acting growth factors, pro-inflammatory cytokines, apoptotic or necrotic cells and microbial products initiate monocytes to differentiate into MPS cell groups. Suppose that when C-PRP containing high-yield monocytes is injected into the local microenvironment of the disease, monocytes are likely to differentiate into M Φ To cause major cell changes.
From monocyte to M Φ In the process of transformation, specific M Φ Phenotype. In the last ten years, a model has been developed, which integrates M Φ The complex mechanism of activation is described as polarization of two opposite states: M Φ Phenotype 1 (M Φ 1, Classic activation) and M Φ Phenotype 2 (M Φ 2, alternative activation). M Φ 1 is characterized by inflammatory cytokine secretion (IFN- γ) And nitric oxide to produce effective pathogen killing mechanism. M Φ The phenotype also produces vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). M Φ The phenotype is composed of anti-inflammatory cells with high phagocytosis. M Φ 2 Produce extracellular matrix components, angiogenesis and chemokines, and interleukin 10 (IL-10). In addition to pathogen defense, M Φ It can also reduce inflammation and promote tissue repair. It is noteworthy that M Φ 2 has been subdivided into M in vitro Φ 2a、M Φ 2b and M Φ 2. It depends on the stimulus. In vivo translation of these subtypes is difficult because the tissue may contain mixed M Φ Groups. Interestingly, based on local environmental signals and IL-4 levels, proinflammatory M Φ 1 can be converted to promote repair M Φ 2。 From these data, it is reasonable to assume that there are high concentrations of monocytes and M Φ C-PRP preparations may contribute to better tissue repair because they have anti-inflammatory tissue repair and cell signal transduction capabilities.
Confused definition of white blood cell fraction in PRP
The presence of white blood cells in PRP treatment bottles depends on the PRP preparation device and may have significant differences. There are many disputes about the existence of leukocytes and their contribution to different sub-PRP products (such as PRGF, P-PRP, LP-PRP, LR-PRP, P-PRF and L-PRF) In a recent review, six randomized controlled trials (evidence level 1) and three prospective comparative studies (evidence level 2) involved 1055 patients, indicating that LR-PRP and LP-PRP had similar safety. The author concluded that the adverse reaction of PRP may not be directly related to the concentration of white blood cells. In another study, LR-PRP did not change the inflammatory interleukin (IL-1) in OA knee β、 IL-6, IL-8 and IL-17). These results support the view that the role of leukocytes in the biological activity of PRP in vivo may come from the crosstalk between platelets and leukocytes. This interaction can promote the biosynthesis of other factors (such as lipoxygen), which can offset or promote the regression of inflammation. After the initial release of inflammatory molecules (arachidonic acid, leukotriene and prostaglandin), lipoxygen A4 is released from activated platelets to prevent neutrophil activation. It is in this environment that M Φ Phenotype from M Φ 1 Switch to M Φ 2。 In addition, there is increasing evidence that circulating mononuclear cells can differentiate into a variety of non-phagocytic cell types due to their pluripotency.
The type of PRP will affect MSC culture. Compared with pure PRP or PPP samples, LR-PRP can induce significantly higher proliferation of bone marrow derived MSCs (BMMSCs), with faster release and better PGF biological activity. All these characteristics are conducive to adding monocytes into the PRP treatment bottle and recognizing their immunomodulatory ability and differentiation potential.
Congenital and adaptive immune regulation of PRP
The most famous physiological function of platelets is to control bleeding. They accumulate at the tissue damage site and the damaged blood vessels. These events are caused by the expression of integrins and selectins that stimulate platelet adhesion and aggregation. The damaged endothelium further aggravates this process, and the exposed collagen and other subendothelial matrix proteins promote the deep activation of platelets. In these cases, the important role of the interaction between von Willebrand factor (vWF) and glycoprotein (GP), especially GP-Ib, has been proved. After platelet activation, platelet α-、 Dense, lysosome and T-granules regulate exocytosis and release their contents into the extracellular environment.
Platelet adhesion molecule
In order to better understand the role of PRP in inflammatory tissues and platelets in immune response, we should understand how different platelet surface receptors (integrins) and junction adhesion molecules (JAM) and cell interactions can initiate critical processes in innate and adaptive immunity.
Integrins are cell surface adhesion molecules found in various cell types and expressed in large quantities on platelets. Integrins include a5b1, a6b1, a2b1 LFA-2, (GPIa/IIa) and aIIbb3 (GPIIb/IIIa). Usually, they exist in a static and low affinity state. After activation, they switch to the state of high ligand binding affinity. Integrins have different functions on platelets and participate in the interaction of platelets with several types of white blood cells, endothelial cells and extracellular matrix. In addition, GP-Ib-V-IX complex is expressed on the platelet membrane and is the main receptor for binding with von vWF. This interaction mediates the initial contact between platelets and exposed subendothelial structures. Platelet integrin and GP complex are related to various inflammatory processes and play an important role in the formation of platelet-leucocyte complex. Specifically, integrin aIIbb3 is necessary to form a stable complex by combining fibrinogen with macrophage 1 antigen (Mac-1) receptor on neutrophils.
Platelets, neutrophils and vascular endothelial cells express specific cell adhesion molecules, called selectin. Under inflammatory conditions, platelets express P-selectin and neutrophil L-selectin. After platelet activation, P-selectin may bind to the ligand PSGL-1 that exists on neutrophils and monocytes. In addition, PSGL-1 binding initiates intracellular signal cascade reaction, which activates neutrophils through neutrophil integrin Mac-1 and lymphocyte function-related antigen 1 (LFA-1). Activated Mac-1 binds to GPIb or GPIIb/IIIa on platelets through fibrinogen, thus stabilizing the interaction between neutrophils and platelets. In addition, activated LFA-1 can combine with platelet intercellular adhesion molecule 2 to further stabilize neutrophil-platelet complex to promote long-term adhesion with cells.
Platelets and leukocytes play a key role in innate and adaptive immune responses
The body can recognize foreign bodies and injured tissues in acute or chronic diseases to initiate wound healing cascade reaction and inflammatory pathway. The innate and adaptive immune systems protect the host from infection, and white blood cells play an important role in overlapping between the two systems. Specifically, monocytes, macrophages, neutrophils and natural killer cells play a key role in the innate system, while lymphocytes and their subsets play a similar role in the adaptive immune system.
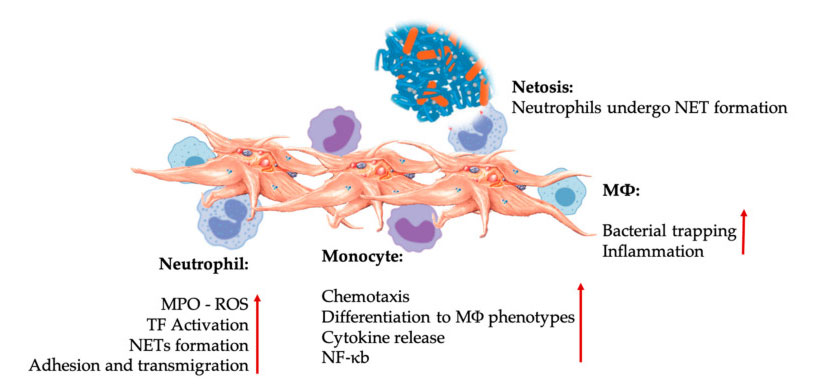
Platelet and leukocyte interactions in innate immune cell interactions. Platelet interacts with neutrophils and monocytes, and finally with M Φ Interact, adjust and increase their effector functions. These platelet-leucocyte interactions lead to inflammation through different mechanisms, including NETosis. Abbreviations: MPO: myeloperoxidase, ROS: reactive oxygen species, TF: tissue factor, NET: neutrophil extracellular trap, NF- κ B: Nuclear factor kappa B, M Φ: Macrophages.
Innate immune system
The role of the innate immune system is to non-specific identify invasive microorganisms or tissue fragments and stimulate their clearance. When certain molecular structures called surface expression pattern recognition receptors (PRRs) combine with pathogen-related molecular patterns and damage-related molecular patterns, the innate immune system will be activated. There are many kinds of PRRs, including Toll-like receptor (TLR) and RIG-1 like receptor (RLR). These receptors can activate the main transcription factor kappa B (NF- κ B) It also regulates multiple aspects of innate and adaptive immune response. Interestingly, platelets also express a variety of immunoregulatory receptor molecules on their surface and cytoplasm, such as P-selectin, transmembrane protein CD40 ligand (CD40L), cytokines (such as IL-1 β、 TGF- β) And platelet-specific TLR.. Therefore, platelets can interact with various immune cells.
Platelet-white cell interaction in innate immunity
When platelets enter or invade blood flow or tissue, platelets are one of the cells that detect endothelial injury and microbial pathogens first. Platelet aggregation and promote the release of platelet agonists ADP, thrombin and vWF, resulting in platelet activation and the expression of platelet chemokine receptors C, CC, CXC and CX3C, thus causing platelets in the infected site or injury.
The innate immune system is genetically predetermined to detect invaders, such as viruses, bacteria, parasites and toxins, or tissue wounds and wounds. It is a non-specific system, because any pathogen will be identified as foreign or non-self and quickly located. The innate immune system relies on a set of proteins and phagocytes, which recognize the well-preserved characteristics of pathogens and quickly activate the immune response to help eliminate invaders, even if the host has never been exposed to specific pathogens before.
Neutrophils, monocytes and dendritic cells are the most common innate immune cells in the blood. Their recruitment is necessary for an adequate early immune response. When PRP is used in regenerative medicine, platelet-white cell interaction regulates inflammation, wound healing and tissue repair. TLR-4 on platelets stimulates platelet-neutrophil interaction, which regulates the so-called leukocyte oxidative burst by regulating the release of reactive oxygen species (ROS) and myeloperoxidase (MPO) from neutrophils. In addition, the interaction between platelet-neutrophil and neutrophil degranulation leads to the formation of neutrophil-extracellular traps (NETs). NETs are composed of neutrophil nucleus and other neutrophil intracellular contents, which can capture bacteria and kill them through NETosis. The formation of NETs is an essential killing mechanism of neutrophils.
After platelet activation, monocytes can migrate to diseased and degenerative tissues, where they carry out adhesion activities and secrete inflammatory molecules that may change chemotaxis and proteolytic properties. In addition, platelets can induce monocyte NF- κ B activation to regulate the effector function of monocytes, which is the key mediator of inflammatory response and activation and differentiation of immune cells. Platelets further promote the endogenous oxidative burst of monocytes to promote the destruction of phagocytic pathogens. The release of MPO is mediated by the direct interaction between platelet-monocyte CD40L-MAC-1. Interestingly, when P-selectin activates platelets under acute and chronic inflammatory tissue conditions, platelet-derived chemokines PF4, RANTES, IL-1 β And CXCL-12 can prevent spontaneous apoptosis of monocytes, but promote their differentiation into macrophages.
Adaptive immune system
After the non-specific innate immune system recognizes the microbial or tissue damage, the specific adaptive immune system will take over. Adaptive systems include antigen-binding B lymphocytes (B cells) and conventional T lymphocytes (Treg) that coordinate the clearance of pathogens. T cells can be roughly divided into helper T cells (Th cells) and cytotoxic T cells (Tc cells, also known as T killer cells). Th cells are further divided into Th1, Th2 and Th17 cells, which have key functions in inflammation. Th cells can secrete proinflammatory cytokines (e.g. IFN- γ、 TNF- β) And several interleukins (e.g., IL-17). They are particularly effective in preventing intracellular virus and bacterial infection. Th cells stimulate the proliferation and differentiation of cells involved in immune response. Tc cells are effector cells, which can eliminate targeted intracellular and extracellular microorganisms and cells.
Interestingly, Th2 cells produce IL-4 and affect M Φ Polarization, M Φ Guided regeneration M Φ 2 Phenotype, while IFN- γ M Φ Change to inflammatory M Φ 1 Phenotype, which depends on the dose and time of cytokines. After IL-4 is activated, M Φ 2 induces Treg cells to differentiate into Th2 cells, and then produces additional IL-4 (positive feedback loop). Th cells convert M Φ The phenotype is directed to the regenerative phenotype in response to biological agents of tissue origin. This mechanism is based on the evidence that Th cells play a significant role in controlling inflammation and tissue repair.
Platelet-white cell interaction in adaptive immunity
The adaptive immune system uses antigen-specific receptors and remembers previously encountered pathogens, and destroys them when it subsequently encounters the host. However, these adaptive immune responses developed slowly. Konias et al. It shows that the platelet component contributes to risk perception and tissue repair, and that the interaction between platelets and leukocytes promotes the activation of adaptive immune response.
During the adaptive immune response, platelets promote monocyte and macrophage responses through DC and NK cell maturation, leading to specific T cell and B cell responses. Therefore, platelet granule components directly affect adaptive immunity by expressing CD40L, a molecule that is essential for regulating adaptive immune response. Platelets through CD40L not only play a role in antigen presentation, but also affect T cell reaction. Liu et al. It was found that platelets regulate CD4 T cell response in a complex way. This differential regulation of CD4 T cell subsets means that platelets promote CD4 T cells to respond to inflammatory stimuli, thus producing strong pro-inflammatory and anti-inflammatory responses.
Platelets also regulate B cell-mediated adaptive response to microbial pathogens. It is well known that CD40L on activated CD4 T cells will trigger CD40 of B cells, providing the second signal required for T-cell-dependent B lymphocyte activation, subsequent allotype conversion, and B cell differentiation and proliferation. In general, the results clearly show the various functions of platelets in adaptive immunity, indicating that platelets connect the interaction between T cells and B cells through CD40-CD40L, thus enhancing the T-cell-dependent B cell response. In addition, platelets are rich in cell surface receptors, which can promote platelet activation and release a large number of inflammatory and biological active molecules stored in different platelet particles, thus affecting the innate and adaptive immune response.
Expanded role of platelet-derived serotonin in PRP
Serotonin (5-hydroxytryptamine, 5-HT) has a clear key role in the central nervous system (CNS), including pain tolerance. It is estimated that most of the human 5-HT is produced in the gastrointestinal tract and then through the blood circulation, where it is absorbed by platelets through serotonin reuptake transporter and stored in dense particles at high concentration (65 mmol/L). 5-HT is a well-known neurotransmitter and hormone that helps regulate various neuropsychological processes in CNS (central 5-HT). However, most of 5-HT exists outside CNS (peripheral 5-HT), and it is involved in regulating the systemic and cellular biological functions of multiple organ systems, including cardiovascular, lung, gastrointestinal, urogenital and platelet functional systems. 5-HT has concentration-dependent metabolism on a variety of cell types, including adipocytes, epithelial cells and white blood cells. Peripheral 5-HT is also a powerful immune modulator, which can stimulate or inhibit inflammation and affect various immune cells through its specific 5-HT receptor (5HTR).
Paracrine and autocrine mechanism of HT
The activity of 5-HT is mediated by its interaction with 5HTRs, which is a superfamily with seven members (5-HT 1 – 7) and at least 14 different receptor subtypes, including the recently discovered member 5-HT 7, its peripheral and function in pain management. In the process of platelet degranulation, activated platelets secrete a large number of platelet-derived 5-HT, which can promote vascular contraction and stimulate the activation of adjacent platelets and lymphocytes through the expression of 5-HTR on endothelial cells, smooth muscle cells and immune cells. Pacala et al. The mitotic effect of 5-HT on vascular endothelial cells was studied, and the potential of promoting the growth of damaged blood vessels by stimulating angiogenesis was determined. How these processes are regulated is not completely clear, but it may involve differential two-way signal pathways in the tissue microcircuit to regulate the functions of vascular endothelial cells and smooth muscle cells, fibroblasts and immune cells through specific 5-HT receptors on these cells. The autocrine function of platelet 5-HT after platelet activation has been described [REF]. The release of 5-HT enhances the activation of platelets and the recruitment of circulating platelets, leading to the activation of signal cascade reactions and upstream effectors supporting platelet reactivity.
Immunomodulatory 5-HT effect
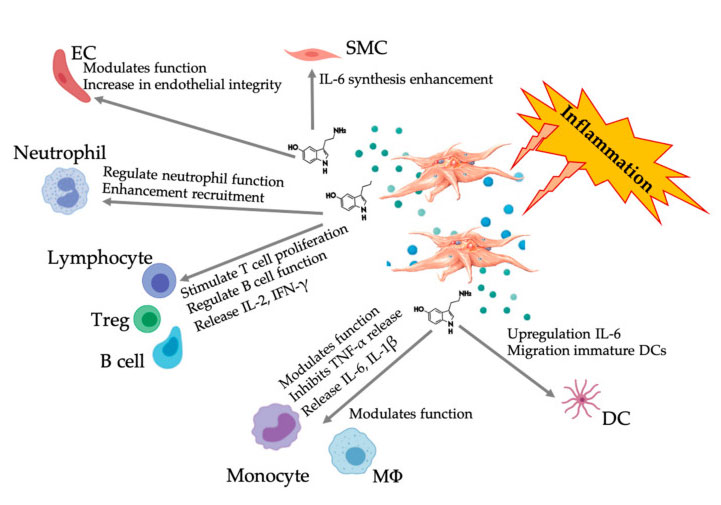
More and more evidence shows that serotonin can play a role in different 5HTR as an immune modulator. According to the 5HTR expressed in various leukocytes involved in inflammatory reaction, platelet-derived 5-HT acts as an immune regulator in both innate and adaptive immune systems. 5-HT can stimulate Treg proliferation and regulate the functions of B cells, natural killer cells and neutrophils by recruiting DC and monocytes to the inflammatory site. Recent studies have shown that platelet-derived 5-HT can regulate the function of immune cells under specific conditions. Therefore, using C-PRP, the platelet concentration is greater than 1 × 10 6/µ L can significantly help to transport the concentration of 5-HT derived from large platelets to the tissue. In the microenvironment characterized by inflammatory components, PRP can interact with several immune cells that play a key role in these pathologies, which may affect clinical results.
Figure displaying the multifaceted 5-HT response after the activation of inflammatory PRP platelets. After the activation of platelets, platelets release their granules, including 5-HT in dense granules, which has a wide range of differential effects on various immune cells, endothelial cells and smooth muscle cells. Abbreviations: SMC: smooth muscle cells, EC: endothelial cells, Treg: conventional T lymphocytes, M Φ: Macrophages, DC: dendritic cells, IL: interleukin, IFN- γ: Interferon γ。 Modified and adapted from Everts et al. and Hull et al.
Analgesic effect of PRP
Activated platelets will release many pro-inflammatory and anti-inflammatory mediators, which can not only cause pain, but also reduce inflammation and pain. Once applied, the typical platelet dynamics of PRP changes the microenvironment before tissue repair and regeneration through a variety of complex pathways related to anabolism and catabolism, cell proliferation, differentiation and stem cell regulation. These characteristics of PRP lead to the application of PRP in various clinical pathological conditions usually associated with chronic pain (such as sports injury, orthopedic disease, spinal disease and complex chronic wound), although the exact mechanism has not been fully determined.
In 2008, Evertz et al. It is the first randomized controlled trial to report the analgesic effect of PRP preparation, which is prepared from the brown layer of the autologous erythrocyte sedimentation rate and activated with autologous thrombin after shoulder surgery. They noted a significant reduction in visual analogue scale scores, the use of opioid based analgesics, and more successful postoperative rehabilitation. It is noteworthy that they reflect the analgesic effect of activated platelets and speculate on the mechanism of platelets releasing 5-HT. In short, platelets are dormant in freshly prepared PRP. After the activation of platelets directly or indirectly (tissue factor), platelets change shape and produce false enough to promote platelet aggregation. Then, they release intracellular α- And dense particles. The tissue treated with activated PRP will be invaded by PGF, cytokines and other platelet lysosomes. More specifically, when dense particles release their contents, they will release a large amount of 5-HT that regulates pain. In C-PRP, platelet concentration is 5 to 7 times higher than that in peripheral blood. Therefore, the release of 5-HT from platelets is astronomical. Interestingly, Sprott et al. The report observed that pain was significantly relieved after acupuncture and moxibustion, the concentration of platelet derived 5-HT was significantly reduced, and then the plasma level of 5-HT was increased.
In the peripheral, platelets, mast cells and endothelial cells will release endogenous 5-HT during tissue injury or surgical trauma. Interestingly, a variety of 5-HT receptors of neurons were detected in the peripheral area, which confirmed that 5-HT can interfere with the nociceptive transmission in the peripheral area. These studies show that 5-HT can affect the nociceptive transmission of peripheral tissues through 5-HT1, 5-HT2, 5-HT3, 5-HT4 and 5-HT7 receptors.
The 5-HT system represents a powerful system that can reduce and increase the degree of pain after harmful stimulation. The central and peripheral regulation of nociceptive signals and changes in the 5-HT system have been reported in patients with chronic pain. In recent years, a large number of studies have focused on the role of 5-HT and its respective receptors in processing and regulating harmful information, resulting in drugs such as selective serotonin reuptake inhibitors (SSRI). This drug inhibits the reuptake of serotonin into presynaptic neurons after the release of serotonin. It affects the duration and intensity of serotonin communication and is an alternative treatment for chronic pain. Further clinical research is needed to clearly understand the molecular mechanism of PRP-derived 5-HT pain regulation in chronic and degenerative diseases.
Other data to solve the potential analgesic effect of PRP can be obtained after the analgesic animal model test. The comparative statistical conclusions in these models are challenging because these studies contain too many variables. Nevertheless, some clinical studies have addressed the nociceptive and analgesic effects of PRP. Several studies have shown that patients receiving treatment for tendinosis or rotator cuff tears have little pain relief. In contrast, several other studies have shown that PRP can reduce or even eliminate the pain of patients with tendon degeneration, OA, plantar fasciitis and other foot and ankle diseases. The final platelet concentration and biological cell composition have been identified as the key PRP characteristics, which help to observe the consistent analgesic effect after the application of PRP. Other variables include PRP delivery method, application technology, platelet activation protocol, biological activity level of PGF and cytokines released, tissue type of PRP application and injury type.
It is noteworthy that Kuffler solved the potential of PRP in alleviating pain in patients with mild to severe chronic neuropathic pain, secondary to the damaged non-regenerative nerve. The purpose of this study is to investigate whether neuropathic pain can be reduced or subsided due to PRP promoting axonal regeneration and target nerve reinnervation. Surprisingly, among the patients receiving treatment, neuropathic pain is still eliminated or alleviated at least six years after surgery. In addition, all patients began to relieve pain within three weeks after the application of PRP.
Recently, similar analgesic PRP effects have been observed in the field of postoperative wound and skin care. Interestingly, the authors reported the physiological aspects of wound pain associated with vascular injury and skin tissue hypoxia. They also discussed the importance of angiogenesis in optimizing oxygenation and nutrient delivery. Their study showed that compared with the control group, patients receiving PRP treatment had less pain and significantly increased angiogenesis. Finally, Johal and his colleagues carried out a systematic review and meta-analysis and concluded that PRP can reduce pain after using PRP in orthopedic indications, especially in patients receiving external epicondylitis and knee OA treatment. Unfortunately, this study did not specify the effects of white blood cells, platelet concentration or the use of exogenous platelet activators, because these variables would affect the overall effectiveness of PRP. The optimal PRP platelet concentration for maximum pain relief is unclear. In the rat model of tendinosis, the platelet concentration was 1.0 × 10 6 / μ At L, the pain can be completely relieved, while the pain relief caused by PRP with half of the platelet concentration is significantly reduced. Therefore, we encourage more clinical studies to investigate the analgesic effects of different PRP preparations.
PRP and angiogenesis effect
C-PRP preparations in precise regenerative medicine allow delivery of biomolecules released by high concentrations of platelets activated at target tissue sites. Therefore, a variety of cascade reactions have been initiated, which contribute to on-site immune regulation, inflammatory process and angiogenesis to promote healing and tissue repair.
Angiogenesis is a dynamic multi-step process involving germination and tissue microvessels from pre-existing blood vessels. Angiogenesis has progressed due to a variety of biological mechanisms, including endothelial cell migration, proliferation, differentiation and division. These cellular processes are prerequisites for the formation of new blood vessels. They are essential for the growth of preexisting blood vessels to restore blood flow and support the high metabolic activity of tissue repair and tissue regeneration. These new blood vessels allow the delivery of oxygen and nutrients, and the removal of by-products from treated tissues.
Angiogenesis activity is regulated by stimulating angiogenic factor VEGF and anti-angiogenic factors (e.g., angiostatin and thrombospondin-1 [TSP-1]). In the diseased and degraded microenvironment (including low oxygen tension, low pH and high lactic acid level), local angiogenic factors will restore angiogenesis activity.
Several platelet soluble media, such as basic FGF and TGF- β And VEGF can stimulate endothelial cells to produce new blood vessels. Landsdown and Fortier reported various results related to the PRP composition, including the intraplatelet sources of many angiogenic regulators. In addition, they concluded that the increase of angiogenesis contributes to the healing of MSK disease in areas with poor vascularization, such as meniscus tear, tendon injury and other areas with poor vascularization.
Promoting and anti-angiogenic platelet properties
In the past few decades, published studies have proved that platelets play a key role in primary hemostasis, clot formation, growth factor and cytokine release, and angiogenesis regulation as part of the tissue repair process. Paradoxically, PRP α- The granules contain a arsenal of pro-angiogenic growth factors, anti-angiogenic proteins and cytokines (such as PF4, plasminogen activator inhibitor-1 and TSP-1), and target the release of specific factors that play a role. Role in angiogenesis. Therefore, the role of PRP in controlling angiogenesis regulation may be defined by the activation of specific cell surface receptors, TGF- β Initiate pro-angiogenic and anti-angiogenic reactions. The ability of platelets to exercise angiogenesis pathway has been confirmed in pathological angiogenesis and tumor angiogenesis.
Platelet-derived angiogenic growth factor and anti-angiogenic growth factor, derived from α- And dense and adhesive molecules. Most importantly, it is generally accepted that the overall effect of platelets on angiogenesis is pro-angiogenic and stimulating. It is expected that PRP therapy will control the induction of angiogenesis, which will contribute to the treatment effect of many diseases, such as wound healing and tissue repair. The administration of PRP, more specifically the administration of high concentration PGF and other platelet cytokines, can induce angiogenesis, angiogenesis and arteriogenesis, because stromal cell-derived factor 1a binds to CXCR4 receptor on endothelial progenitor cells. Bill et al. It is suggested that PRP increases ischemic neovascularization, which may be due to stimulation of angiogenesis, angiogenesis and arteriogenesis. In their in vitro model, endothelial cell proliferation and capillary formation were induced by a large number of different PDGs, of which VEGF was the main angiogenic stimulator. Another important and essential factor for restoring the angiogenesis pathway is the synergy between multiple PGFs. Richardson et al. It was proved that the synergistic activity of angiogenic factor platelet-derived growth factor-bb (PDGF-BB) and VEGF led to the rapid formation of mature vascular network compared with the activity of individual growth factor. The combined effect of these factors was recently confirmed in a study on the enhancement of cerebral collateral circulation in mice with long-term hypoperfusion.
Most importantly, an in vitro study measured the proliferative effect of human umbilical vein endothelial cells and various platelet concentrations on the selection of PRP preparation device and platelet dose strategy, and the results showed that the optimal platelet dose was 1.5 × 10 6 platelets/ μ 50. To promote angiogenesis. Too high platelet concentration may inhibit the angiogenesis process, so the effect is poor.
Cell aging, aging and PRP
Cell senescence can be induced by various stimuli. This is a process in which cells stop dividing and undergo unique phenotypic changes to prevent the unrestricted growth of damaged cells, which plays an important role in the prevention of cancer. In the process of physiological aging, cell replication aging will also promote cell aging, and the regeneration ability of MSCs will be reduced.
Effects of aging and cell aging
In vivo, many cell types will age and accumulate in various tissues during aging, among which there are a large number of aging cells. The accumulation of aging cells seems to increase with the increase of age, immune system damage, tissue damage or stress-related factors. The mechanism of cellular aging has been identified as the pathogenic factor of age-related diseases, such as osteoarthritis, osteoporosis and intervertebral disc degeneration. A variety of stimuli will aggravate cell aging. In response, the senescence-related secretory phenotype (SASP) will secrete high concentrations of protein cells and cytokines. This special phenotype is related to aging cells, in which they secrete high levels of inflammatory cytokines (such as IL-1, IL-6, IL-8), growth factors (such as TGF- β、 HGF, VEGF, PDGF), MMP, and cathepsin. Compared with young people, SAPS has been proved to increase with age, because the steady-state process is destroyed, resulting in cell aging and reduced regeneration ability. Specifically, in joint diseases and skeletal muscle diseases. In this regard, immune aging is considered to be a significant change in the secretion spectrum of immune cells, indicating that the concentration of TNF-a, IL-6 and/or Il-1b increases, leading to low-grade chronic inflammation. It is worth noting that stem cell dysfunction is also related to non-cellular autonomous mechanisms, such as aging cells, especially the production of pro-inflammatory and anti-regenerative factors through SASP.
On the contrary, SASP can also stimulate cell plasticity and reprogramming of adjacent cells. In addition, SASP can organize the communication with various immune mediators and activate immune cells to promote the clearance of aging cells. Understanding the role and function of aging cells will contribute to the healing and tissue remodeling of MSK muscles and chronic wounds.
It is noteworthy that Ritcka et al. An extensive study was carried out, and the main and beneficial role of SASP in promoting cell plasticity and tissue regeneration was discovered, and the concept of transient treatment delivery of aging cells was introduced. They cautiously mentioned that aging is mainly a beneficial and regenerative process.
Cell aging and potential of PRP
As the number of stem cells decreases, aging will affect the performance of stem cells. Similarly, in humans, stem cell characteristics (such as dryness, proliferation and differentiation) also decrease with age. Wang and Nirmala reported that aging would reduce the characteristics of tendon cell stem cells and the number of growth factor receptors. An animal study showed that the concentration of PDGF in young horses was high. They concluded that the increase in the number of GF receptors and the number of GF in young individuals may have a better cellular response to PRP treatment than older individuals in young individuals. These findings reveal why PRP treatment may be less effective or even ineffective in elderly patients with fewer stem cells and “poor quality”. It has been proved that the aging process of aging cartilage is reversed and the resting period of chondrocytes is increased after PRP injection. Jia et al. It is used to study mouse dermal fibroblasts in vitro photoaging, with and without PRP treatment, to clarify the mechanism of PGF counteraction in this model. PRP group showed a direct effect on extracellular matrix, increased type I collagen and decreased the synthesis of metalloproteinases, indicating that PRP can counteract cell aging, and also in degenerative MSK disease.
In another study, PRP was used to collect aged bone marrow stem cells from aged mice. It has been determined that PRP can recover a variety of stem cell functions from aging, such as cell proliferation and colony formation, and reconstruct the markers related to cell aging.
Recently, Oberlohr and his colleagues extensively studied the role of cell aging in weakening muscle regeneration, and evaluated PRP and platelet-poor plasma (PPP) as biological treatment options for skeletal muscle repair. They envisioned that PRP or PPP treatment for skeletal muscle repair would be based on biological factors customized for SASP specific cell markers and other factors that lead to fibrosis development.
It is reasonable to believe that before the application of PRP, targeted cell aging can improve the regeneration characteristics of biological treatment efficacy by reducing local SASP factors. It has been suggested that another option to improve the results of PRP and PPP treatment for skeletal muscle regeneration is to selectively remove aging cells with aging scavengers. There is no doubt that the recent research results on the effect of PRP on cell aging and aging are fascinating, but they are still in the initial stage. Therefore, it is unreasonable to make any suggestions at this time.
(The contents of this article are reprinted, and we do not provide any express or implied guarantee for the accuracy, reliability or completeness of the contents contained in this article, and are not responsible for the opinions of this article, please understand.)
Post time: Mar-01-2023